Oral Presentation Annual Meetings of the Endocrine Society of Australia and Society for Reproductive Biology and Australia and New Zealand Bone and Mineral Society 2016
Pembrolizumab-induced thyroiditis in patients with metastatic melanoma: A novel form of autoimmune thyroid disease (#259)
Introduction: Pembrolizumab is a fully human monoclonal antibody directed against programmed cell death 1, targeting the effector arm of the immune checkpoint pathway1. Pembrolizumab has superseded other immunotherapies as first-line treatment in patients with metastatic melanoma2 however is associated with autoimmune endocrinopathies. The most common of these is thyroid dysfunction, with reported incidence <1-16%3,4, but evidence is limited regarding its presentation and management.
Methods: Retrospective review of patients with metastatic melanoma who received pembrolizumab 2mg/kg intravenously every three weeks through compassionate access, enrolled from November 2013-August 2015. All patients were evaluated at the Chris O’Brien Lifehouse and Department of Endocrinology, Royal Prince Alfred Hospital.
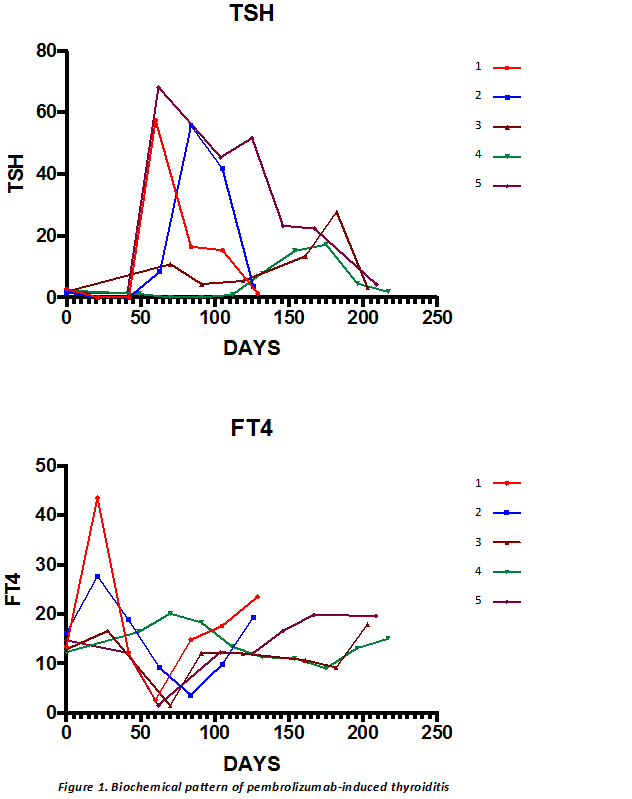
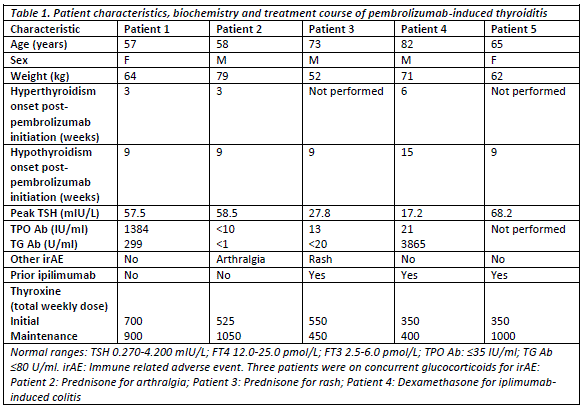
Results: 41 patients were identified. Median age was 65 (range: 37-90) years and 32 (78%) were male. Median number of pembrolizumab-cycles received was 4 (1-20), with treatment ongoing in 15 patients (37%). Only 15 (37%) of patients were ipilimumab-naïve. Immune-related adverse events (irAEs) occurred in 21 (51%) patients: most commonly dermatological (24%) and rheumatological (22%). Hypophysitis was observed in 1 patient but onset correlated with previous ipilimumab. Primary thyroiditis was confirmed in 5 (12%) patients via baseline TFTs and anti-thyroidal antibodies, characterised by asymptomatic hyperthyroidism followed by symptomatic hypothyroidism (figure 1), requiring ongoing thyroxine replacement. Patient characteristics, investigations and treatment are summarised in table 1.
Conclusion: Pembrolizumab-induced thyroiditis was characterised by two distinct phases: hyperthyroidism and hypothyroidism by 3 and 9 weeks respectively, post-initiation of pembrolizumab. Considering that the hyperthyroid phase was asymptomatic, and that exposure to radiocontrast, concurrent glucocorticoid therapy for other irAEs or sick euthyroid condition may result in mild biochemical hyperthyroidism, patients should be followed-up closely. Given the persistence and degree of hypothyroidism, patient age and co-morbidities should not preclude initiation of standard (rather than conservative) thyroxine replacement therapy at the onset of biochemical hypothyroidism in these patients, to prevent symptomatic hypothyroidism.
- Ribas. Tumour immunotherapy directed at PD-1. N Eng J Med. 2012;366(26):2517-9.
- Robert et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Eng J Med. 2015;372(26):2521-32
- Mace et al. Thyroid abnormalities during anti-PD1 cancer immunotherapy. Endocrine Abstracts. 2015;38:443
- Topalian S et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med. 2012;366:2443-2454