Oral Presentation Annual Meetings of the Endocrine Society of Australia and Society for Reproductive Biology and Australia and New Zealand Bone and Mineral Society 2016
Comparing the analytical utility of the modern Thyroid Stimulating Immunoglobulin (TSI) assays in a case of TSH receptor antibody negative Graves’ Disease (#263)
Endocrinologists occasionally encounter cases of Graves’ disease (GD) with undetectable TSH Receptor Antibody (TRAb). In the past, this could be analysed on the cell based Thyroid Stimulating Immuoglobulin-cyclic AMP assay (TSI-cAMP). However, the complexity of maintaining a cell line and the push for automation has led to the withdrawal of this assay from regular diagnostic laboratories. In recent years, genetically engineered semi-quantitative TSI assays like Siemens Immulite®2000 TSI assay (I-2000TSI) and a genetically modified cell based cAMP assay, Thyretain®, both received FDA approval as tests specific for TSI. Here we present a case of TRAb negative GD to illustrate the utility and limitation of the new analytical platforms.
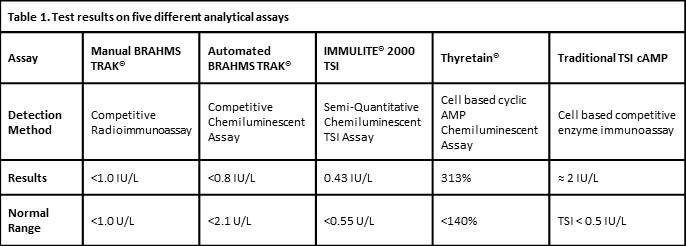
A 42 year old female referred to the RPAH Endocrine Clinic for management of GD in April 2012 was grossly thyrotoxic with Graves’ ophthalmopathy. GD was confirmed on thyroid scan with uptake of 7.9% (RR 1-5%) and manual BRAHMS® TRAb 16U/L (RR≤1.0). She was commenced on carbimazole 20mg daily and became biochemically euthyroid one month later. She had four flares of biochemical thyrotoxicosis over 2 years but declined recommendation for definitive therapy. TRAb became undetectable in June 2014 and carbimazole was reduced to 5mg daily in October 2014. Four months later, she had another flare with undetectable TSH, FT4 of 25.3pmol/L (RR9-19) and FT3 of 8.3pmol/L (RR2.6-6.0). Although TRAb remained undetectable on conventional competitive immunoassays, carbimazole had to be increased to 25mg daily to maintain euthyroidism. The serum was tested concurrently on I-2000TSI, Thyretain® and TSI-cAMP. I-2000TSI reported negative TSI level of 0.43U/L (RR<0.55), whereas Thyretain® and TSI-cAMP confirmed TSI activities consistent with the clinical picture, at 313% and 400% above normal cut off, respectively.
In conclusion, in this case, Thyretain® had proven efficient capability in confirming disease activity in GD while I-2000TSI did not.