Poster Presentation Annual Meetings of the Endocrine Society of Australia and Society for Reproductive Biology and Australia and New Zealand Bone and Mineral Society 2016
High-dose denosumab provides symptom control in familial expansile osteolysis (#413)
Introduction: Familial expansile osteolysis (FEO) is an autosomal dominant disorder characterised by generalised bony modelling abnormalities, focal osteolytic lesions and elevated bone turnover. This can result in bone pain, fracture/deformity and onset of conductive deafness and dental disease in childhood. FEO is caused by a duplication mutation in the TNFRSF11A gene, which encodes receptor activator of nuclear factor-K B (RANK), and results in increased RANK activity. Denosumab is an anti-RANK ligand monoclonal antibody that specifically targets the underlying biochemical defect in FEO. However, the efficacy of denosumab in FEO has not been previously reported.
Case: We report a 45 year old man who emigrated from Northern Ireland with an autosomal dominant family history of FEO. He reported development of conductive deafness and resorption of teeth during his teenage years. He re-presented at age 43 with severe left knee pain and reduced mobility. Imaging showed an expansile lesion of the left distal femur with associated hyperaemia. There was high tracer activity at this site on technetium-99m-methylene diphosphate bone scan. Femoral bone histopathology showed features of high bony turnover and both alkaline phosphatase and C-terminal telopeptide of type 1 collagen were elevated. These clinical and biochemical features and family history are consistent with FEO and genetic testing for a mutation in the TNFRSF11A gene is underway.
He had received pamidronate ten years ago for milder bilateral knee pain with only modest effect. The patient was prescribed risedronate for 3 months which reduced bony turnover markers (Figure) but did not attenuate knee pain. Subsequently, he was prescribed subcutaneous denosumab 120mg monthly. Denosumab reduced pain, improved mobility and quality of life and resulted in further reduction in markers of bone turnover (Figure).
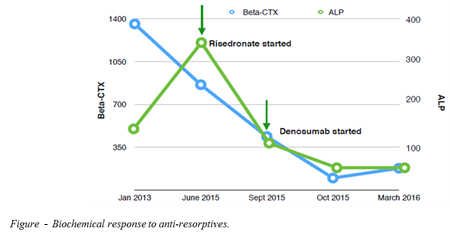
Conclusions: Denosumab shows potential as a targeted disease-modifying therapy in FEO. Further studies are required to optimise denosumab dosing and treatment duration in this rare condition.