Poster Presentation Annual Meetings of the Endocrine Society of Australia and Society for Reproductive Biology and Australia and New Zealand Bone and Mineral Society 2016
Day-to-day consistency was observed in the greater early glucose-lowering effect of faster-acting insulin aspart (#374)
Background and aims: faster-acting insulin aspart (faster aspart) is insulin aspart (IAsp) in a new formulation with faster initial absorption and greater early glucose-lowering effect. This study aimed to investigate within-subject day-to-day variability in the pharmacodynamic (PD) effect of faster aspart.
Materials and methods: subjects with type 1 diabetes (n=45; mean HbA1c [±SD]: 7.4±0.85%; mean age [±SD]: 44.0±10.4 years) received three single 0.2 U/kg doses of either faster aspart (n=23) or IAsp (n=22) under automated euglycaemic glucose-clamp conditions (ClampArt, blood glucose target 5.5 mmol/l; duration up to 12 h post-dose) on 3 identical study days in a double-blind, randomised fashion.
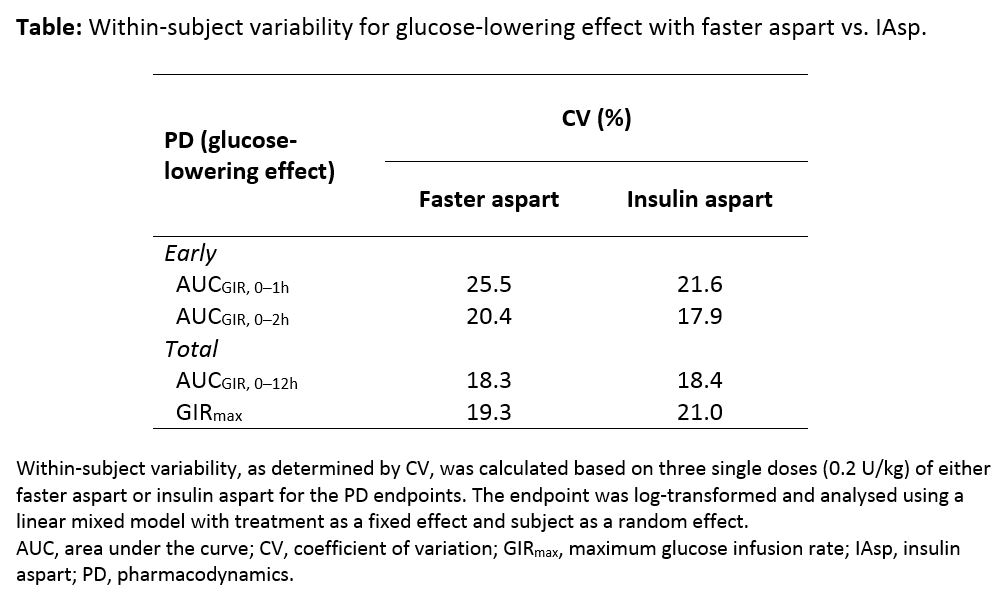
Results: compared with IAsp, faster aspart had a greater early PD effect, indicated by 11–28% higher glucose infusion rates (GIRs) in the first 2 h (treatment ratio [95% CI]: AUCGIR, 0–1h, 1.28 [1.17; 1.41]; AUCGIR, 0–2h, 1.11 [1.03; 1.20]) with low intra-individual day-to-day variability. Within-subject coefficients of variation for early glucose-lowering effects were low for faster aspart and similar to IAsp (Table), as was within-subject variability for total GIR (AUCGIR, 0–12h) and maximal GIR (GIRmax; Table).
Conclusion: the higher early glucose-lowering effect of faster aspart is consistent and supported further by low day-to-day variability in PD endpoints.